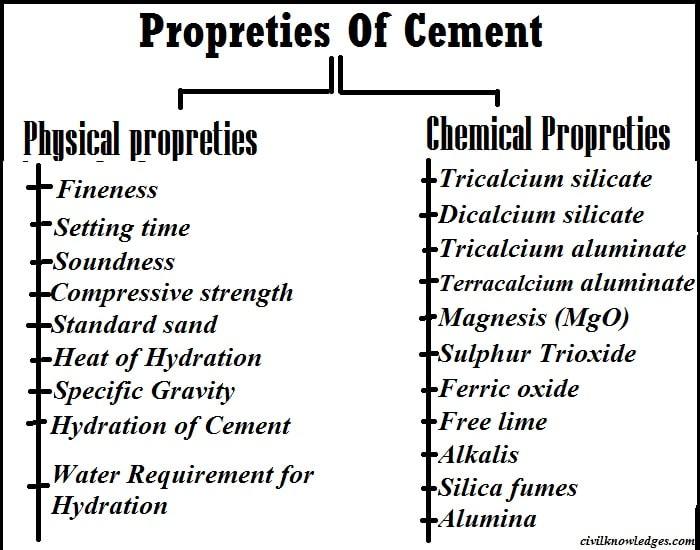
Cement is used to make mortar or concrete and helps it reach strength after the final set. Good cement can provide higher resistance to mortar or concrete and help resist moisture and extend the structure’s life. Therefore, before understanding mortar or concrete or their properties and uses, one must first understand the properties of cement. Here, we discussed the properties of cement. The properties of cement can be divided into two categories, the first being the physical properties of cement, and the second being the chemical properties of cement. The cement performance mainly depends on its composition, raw materials, and the attention paid during the combustion and grinding process.
Physical Properties of Cement
Various cement mixtures used in construction have their physical properties. Some of the main parameters control the quality of cement. The physical properties of high-quality cement depend on:
- Soundness
- Strength
- Fineness
- Consistency
- Heat of hydration
- Setting time
- Bulk density
- Specific gravity
- Loss of ignition
Soundness of cement
The soundness of cement is the ability to maintain the volume of the hardened paste after setting. If the cement has undergone a delayed destructive expansion, it is unhealthy (i.e., lack of strength).
The lack of cement is due to an excess of free lime or burnt magnesium oxide. Cement’s hardness refers to the quality that should extend into the environment. Cement has low hardness and will expand when it hardens and cause cracks in the structure.
The main factor that causes weakness is excessive lime or quicklime in the cement production process, which causes the lime to oxidize to form calcium carbonate. The size of calcium carbonate is more extensive, which leads to expansion and expansion. Crack.
We may all be familiar with the idea of gypsum as a retarder, as gypsum is added to cement under the condition of tricalcium aluminate (C3A), also known as diatomaceous earth. If there is a lot, then the gypsum will delay the fixation and eventually show the concrete’s slow expansion.
Strength of cement
A cement mortar’s strength is usually determined in three ways: compression, tension, and bending. These strengths will be influenced by many factors, including water-to-cement ratio, total cement end ratio, type and classification of fine aggregate, sample mixing and formation method, polymerization conditions, sample size and shape, moisture content test time, loading conditions, and service life.
Since cement gains strength over time, it is necessary to determine when to test the strength. Usually, the time is one day (for early high strength cement), three days, seven days, 28 days, and 90 days (for heat reduction of hydrated cement). When considering the strength test of cement mortar, two factors must be considered:
The strength of cement mortar is not directly related to the strength of concrete. The strength of cement mortar is usually used as a quality control measure.
The resistance test is performed on the cement mortar (cement + water + sand) and not on the cement mortar.
Fineness of Cement
Cement fineness is a measure of cement particles’ size and is expressed by the cement’s specific surface area. Purity may be calculated by particle size analysis (sieve analysis), air permeability method, or sedimentation method. Sieve analysis measures concrete particle size, while air permeability and sedimentation methods measure a given surface area.
Since cement particles are very fine (less than 90 μm), sieve analysis is not suitable for cement. Because of this shortcoming, the cement’s smoothness is always measured by the air permeability method and is expressed by the specified surface area.
For a specific weight of cement, fine cement has a larger surface than coarse cement because the specified surface area is inversely proportional to the particle size.
According to previous reports, if the cement is finer, its specific surface area will be enormous. The finer the cement, the greater its surface area, and the greater the surface area for chemical reactions with water, thus increasing the wetting rate, which leads to the early development of resistance. But the final strength will not be affected.
Importance/Effects of cement Fineness
The cement finest affects the hydration rate and, thus, the quality of increase in electrical resistance.
Consequently, the greater the area available per unit volume for the interaction between water and cement.
Therefore, the fine cement reacts faster with water, and the development speed of electrical resistance and the corresponding heat to wetting is high.
Purity is a measure of individual particles’ state, which results in a larger surface area and greater surface area of adhesion.
You can reduce bleeding by increasing smoothness. However, higher purity may also result in more water being required for processability, which may increase the potential for shrinkage.
The fineness test is used to check the correct grinding of cement and cementitious grinding.
Consistency of cement
The consistency of cement slurry is defined as the percentage of water required by the cement slurry. The viscosity of the mortar can determine the amount of water needed to prepare the slurry. Since the amount of water affects the setting time of the cement, the consistency must be determined.
Consistency is the ability to resist shear deformation. This means that the concrete is holding together and not moving. Consistency plays a vital role in determining the compressive strength of concrete or testing the workability of concrete. It takes a certain quantity of water to react with the cement to produce a paste with sufficient elasticity. A small amount of water will not complete the chemical reaction, which will lead to a decrease in the electrical resistance. In contrast, more water will increase the ratio of water to cement, thus reducing its electrical resistance. The fineness of the particles directly affects the consistency of the cement.
Heat of hydration
In the hydration process, the heat generated when cement and water interact is called the heat of hydration. The water temperature is influenced by the ratio of C3S and C3A in the cement, but it is also affected by the water to cement ratio, the smoothness, and the hardening temperature.
In large concrete structures, water heat is generated much faster than it is dissipated (especially in the center of large concrete blocks), causing the temperature to rise in the center of these large concrete blocks at a high temperature. When the concrete cools to room temperature, unnecessary pressure is created. On the contrary, the heat of the water can help in maintaining proper hardening temperature in winter.
Read Also: What are the 5 Stages of Cement Hydration Process
Setting time of cement
When we mix cement with water, it will moisten and form a cement slurry. Due to its plasticity, putty can be shaped into any desired shape. During this time, the cement continues to interact with the water, and slowly the cement begins to lose elasticity and begins to harden. This whole process is called cement setting time.
Initial setting time
The time during which the cement can be shaped into any desired shape without losing the initial setting time of the cement is called.
Final setting time
When the cement completely loses its elasticity and hardens is the time of the final set of the cement.
Loss of ignition
Loss of ignition is the loss of weight resulting from raising the material’s temperature to a predetermined level. As an indicator, it can be used to monitor and improve the quality of the final product.
Weight loss during heating may be due to evaporation or volatilization of various sample components. Water would be lost at 100-105 ° C, organic matter would burn at around 550 ° C, and most carbonate would be lost between 800-1000 ° C.
Bulk density
When cement is mixed with water, water replaces areas where there is usually air. Therefore, the bulk density of cement is not so important. Cement has a variable density range, depending on the percentage of cement formation. The density of concrete can vary from 62 to 78 pounds per cubic foot.
Specific gravity
Usually, specific gravity is used in calculating the mixture ratio. The specific gravity of Portland blast furnace slag and Portland-Pozzolan strain can have a specific gravity close to 2.90, while the specific gravity of Portland cement is typically around 3.15.
Chemical properties of cement
Limestone (calcium), sand, or clay (silicon), and bauxite (aluminum) are the raw materials to produce cement. Chemical analysis of cement raw materials provides information about the chemical properties of cement.
Tricalcium aluminate (C3A)
The low content of C3A makes the cement resistant to sulfates. Gypsum reduces C3A hydration and releases a lot of heat in the early stages of hydration. The power provided by C3A does not exceed a small amount.
Type I cement: contains up to 3.5% SO3 (in cement with C3A greater than 8%). Type II cement: 3% SO3 (in cement with C3A less than 8%)
Tricalcium silicate cement (C3S)
C3S causes rapid hydration, hardening and also responsible for the initial strength of the cement under the initial settings.
Dicalcium silicate (C2S)
Unlike tricalcium silicate, which helps increase strength early, silicon silicate in cement helps increase strength after a week.
Ferrite (C4AF)
Ferrite is a melting agent. It reduces the melting temperature of ingredients in the oven from 3000 ° F to 2,600 ° F. Although it moisturizes quickly, it does not contribute much to the strength of the cement.
Magnesia (MgO)
The Portland cement production process uses magnesium oxide as the raw material for dry process plants. Too much magnesium oxide will make the cement not stiff and swollen, but a small amount of magnesium oxide will increase the cement’s strength. Cement production based on magnesium oxide can also reduce carbon dioxide emissions. The MgO content for all types of cement is limited to 6%.
Sulfur trioxide
Excessive sulfur trioxide can make the cement unable to bond.
Iron oxide/iron oxide
In addition to the increased strength and hardness, iron oxide or iron trioxide is mainly responsible for the color of the cement.
Alkalis
The amount of potassium oxide (K2O) and sodium oxide (Na2O) determines the alkaline content of the cement. Cement with a large amount of alkali may cause some difficulties in adjusting cement setting time. Low alkaline cement, the use of calcium chloride in concrete can cause discoloration. In lime slag cement, blast furnace slag is not itself hydraulic but is “activated” by adding alkali. The total alkali content calculated from the formula Na2O + 0.658 K2O has an optional limit of 0.60%.
Free lime cement
Sometimes the free lime in the concrete causes swelling.
Cement Silica fumes
Silica fumes are added to cement to improve various properties, especially pressure resistance and bonding strength. Although adding silicon smoke will prolong the hardening time, it can guarantee too high electrical resistance. Therefore, Portland cement containing 5-20% silica fume is usually produced for Portland cement projects that require high strength.
Cement Alumina
Cement with high alumina content has the ability to withstand harsh temperatures because alumina is chemical resistant. It can also speed upsetting, but it will weaken the concrete.